Which of the Following Bonds Is Least Polar
SOMEONE ASKED Which of the following bonds is LEAST polar. C-Se CO Cl-Br OO N-H C-H.

Bond Polarity Electronegativity And Dipole Moment Chemistry Practice Problems Youtube
Which of the following bonds is least polar.

. C-O is a polar covalent bond. HERE THE ANSWERS Br-Br is a nonpolar covalent bond. Atoms ability to attract and hold electrons.
DIEN of C-N049non-polar 0517polar. See full answer below. Covalent bond in which one pair of electrons is shared by two atoms.
44K views View upvotes Answer requested by Greg Jeffries Elena Ursa and 1 more. So the least polar bond that is still polar is C-Cl. Which of the following bonds is the least polar.
Two very e- atoms undergo ionic bonding e. If atom X forms a diatomic molecule with itself the bond is ________. C - C 347 kJmol.
A Ionic bonding results from the transfer of electrons from one atom to another. C C 614 kJmol. A very e- atom and a weakly e- atom are covalently bound d.
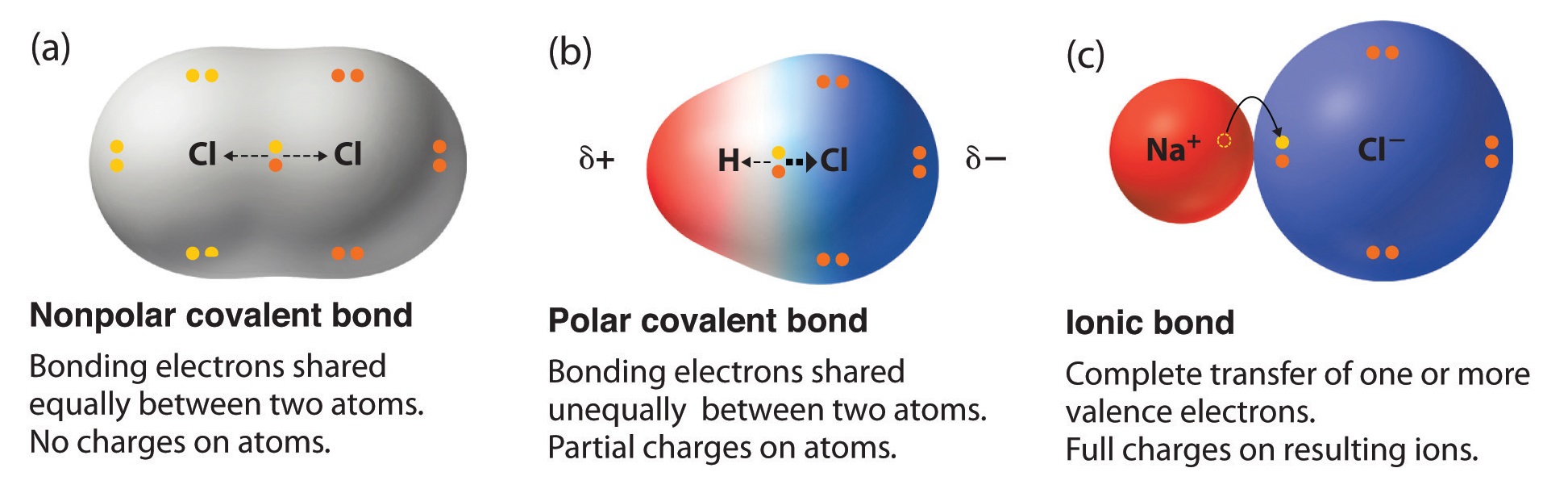
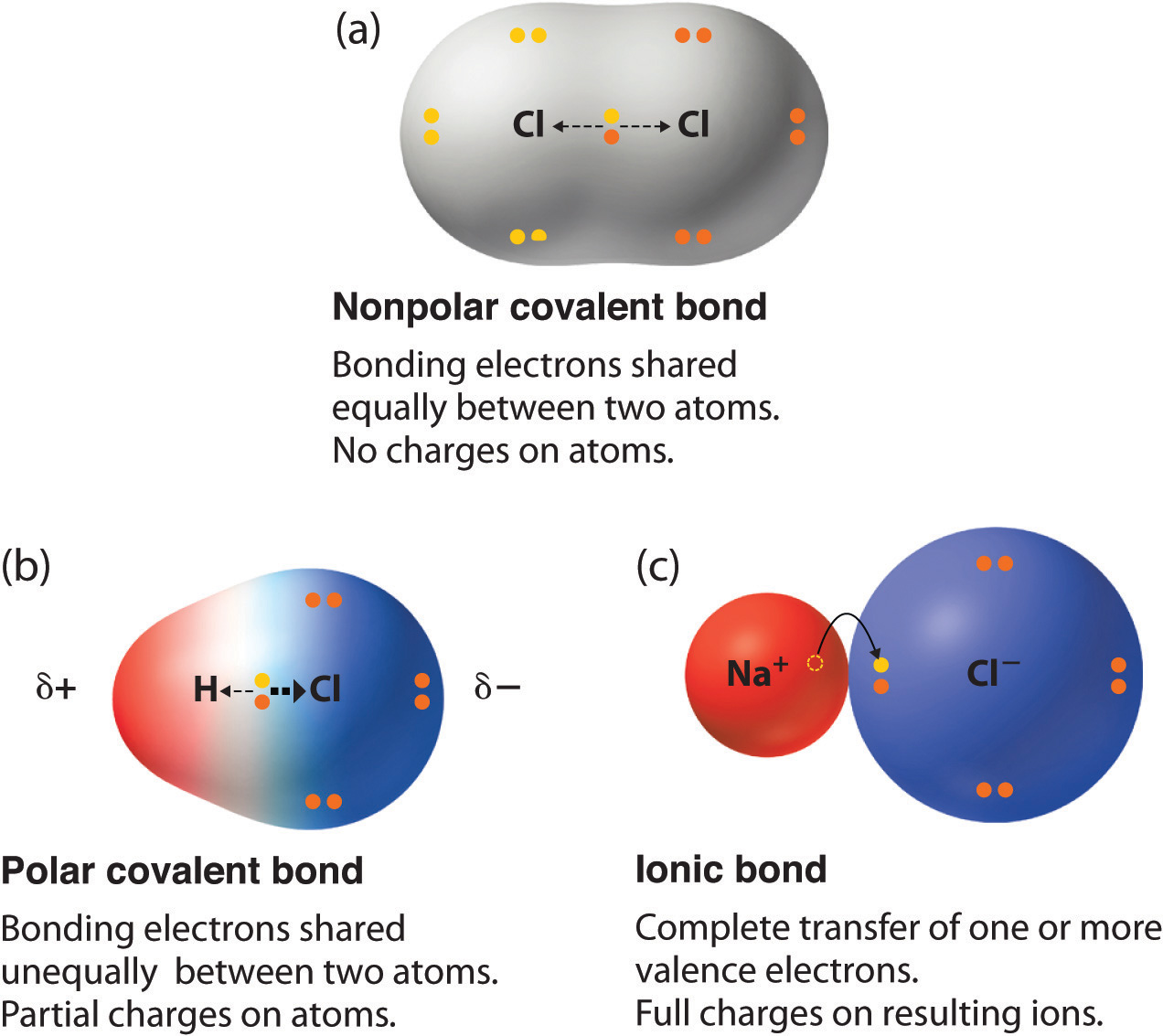
H - O. B Dipole moments result from the unequal distribution of electrons in a molecule. The difference in electronegativities of two elements can be used to predict the nature of the chemical bond.
Br-Br is the most nonpolar because it is a bond. C The electrons in a polar bond are found nearer to the more electronegative element. Non polar covalent bond.
A covalent bond in which the bonding electrons are equally attracted to both bonded atoms. Which of the following bonds would be the most polar without being considered ionic. A CO b HC c PCl d NaCl e They are all nonpolar.
H-C is a nonpolar covalent bond but not as nonpolar as Br-Br. EqH - 21 N - 30 O - 35 B - 15 Cl - 30 F -. We begin by using the electronegativity values to determine the least polar bonds.
Select the answer choice that correct identifies if the bonds formed would be nonpolar covalent polar covalent or ionic. Polar bonds form between any two dissimilar atoms that have different electronegativity values. A CO b HC c PCl d NaCl e They are all nonpolar.
Arrange the bonds from most to least polar. The least polar would be Br-Br because it is the same element therefore there is no element attracting more electrons than the other. C - Cl.
None of the above. Less than 17 the bond is usually covalent less than 05 the bond has some degree of polarity. NO has the least polar bond.
Using your periodic table you can locate each element and see the distance between the two and generally the further apart they are the more polar the bond will be. Two weakly e- atoms undergo ionic bonding b. When differences are 17 or greater the bond is usually ionic.
The number of polar covalent bonds in NH3 is ____. The polarity of a bond describes how evenly or unevenly the bonding electrons are shared between two atoms. If one atom is significantly more.
A HF B HCl C HO D HS Medium Solution Verified by Toppr Correct option is D HS has least polarity in the bond because the electronegativity difference between H and S is the least. Covalent bond in which two pairs of electrons are. S-Cl is a polar covalent bond.
Which of the following statements is incorrect. And less than 05 are considered to be nonpolar. Given the following bond energies estimate the enthalpy change heat of reaction for the combustion of methane CH 4.
B - F. Try to use a. H 2 O.
Which of the following has least polarity in bond. Solve any question of Chemical Bonding and Molecular Structure with- Patterns of problems. A CO b HC c PCl d NaCl e They are all nonpolar.
Which of the following molecules has a dipole moment. The least non-polarpolarionic bond is C-C obviously. Which of the following bonds is least polar.
C-H is non-polar because the have negligible difference in electronegativity. Mg-O C-O O-O Si-O N-O. Two very e- atoms are covalently bound c.
Which is the least polar bond. Na - O. Which of the following statements best describes a relatively polar bond.
Which of the following bonds is least polar. D A molecule with very polar bonds can be. C-Se C-O and N-H are polar while Cl-Cl OO are non-polar because they have identical bonded atoms.

Covalent Bonds Biology For Majors I
8 7 Bond Polarity And Electronegativity Chi Chemistry Libretexts

No comments for "Which of the Following Bonds Is Least Polar"
Post a Comment